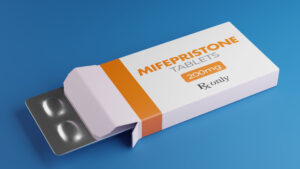
On the eve of a government shutdown, the U.S. Food and Drug Administration (FDA) quietly approved a new generic version of the chemical abortion pill mifepristone—despite promising a full safety review just weeks earlier.
The approval, granted to Virginia-based Evita Solutions, marks the second generic version of the abortion drug on the U.S. market. The brand-name Mifeprex has been available since 2000, and another generic was approved in 2019.
This latest move has ignited outrage from pro-life leaders, medical experts, and members of Congress who argue the FDA is ignoring mounting evidence of the dangers chemical abortion pills pose to women.
Mounting Evidence of Harm
A recent study by the Ethics and Public Policy Center revealed that nearly one in nine women who take mifepristone suffer serious complications within 45 days. Reported risks include sepsis, hemorrhage, blood transfusions, infection, and emergency surgery.
Pennsylvania has already seen the tragic consequences of these drugs. In past reporting, PA Family Institute highlighted how women in our state were hospitalized after taking the abortion pill regimen. In one documented case, a Pennsylvania woman required emergency surgery following severe bleeding. These are not isolated incidents—they are part of a growing pattern of harm too often hidden by the abortion industry’s assurances of “safety.”
Critics Speak Out
Reaction to the FDA’s move was swift.
- Ryan T. Anderson, president of the Ethics and Public Policy Center, wrote on X: “The FDA just made a giant mistake in approving another generic form of the chemical abortion pill, all while promising Senators that it would be conducting a thorough review of the safety. Why approve a generic before conducting the safety review?”
- Lila Rose of Live Action blasted the decision, posting: “UNACCEPTABLE: The FDA just approved another generic of the abortion pill mifepristone. This drug starves babies and harms mothers! The FDA just said it would do a new serious safety study—so why approve another generic now? @RobertKennedyJr must reverse this decision!”
- The March for Life organization warned: “The FDA’s decision to approve this drug is deeply concerning. Women deserve real, compassionate healthcare that prioritizes their well-being, not assurances that a dangerous chemical abortion pill is ‘safe.’ This is just making a dangerous drug even more accessible.”
Sen. Josh Hawley called the move “shocking,” noting that chemical abortion drugs are “dangerous and even deadly for the mother. And of course 100% lethal to the child.”
A Political Bait-and-Switch
What makes this approval particularly troubling is its timing. Less than two weeks earlier, Health and Human Services Secretary Robert F. Kennedy Jr. and FDA Commissioner Marty Makary had pledged in a letter to state attorneys general that the agency would re-examine mifepristone’s safety record. They acknowledged that previous administrations had loosened restrictions on the drug without “adequate consideration.”
Yet before any review was completed, the FDA expanded access by approving another generic. Even more concerning, the FDA did not issue its usual press release—suggesting the agency anticipated backlash.
A Company Bent on “Normalizing” Abortion
Evita Solutions, the company behind the newly approved generic, openly states its mission is to “normalize abortion” and make it “accessible to all.” This radical agenda ignores the mounting evidence of harm to women, and instead treats abortion as a product to be marketed as widely and cheaply as possible.
The Pennsylvania Connection
Chemical abortions already make up nearly two-thirds of abortions in the U.S., and Pennsylvania has not been spared. Reports from across the state have shown how mail-order abortion pills have placed women at serious risk while bypassing in-person medical oversight. Women who experience severe bleeding or infection are often left to seek emergency care without telling doctors they took abortion drugs, a tactic the abortion industry itself has encouraged. This is already happening here in Pennsylvania.
This creates a dangerous cycle: complications go unreported, data appears safer than reality, and the FDA points to incomplete information to justify more approvals.
Where Do We Go From Here?
Instead of conducting the promised safety review, the FDA has sadly further opened the floodgates for chemical abortion. Women’s lives—and the lives of their unborn children—are the ones placed at risk.
Pennsylvanians must not forget what this means for our state. Every expansion of chemical abortion availability means more women here will face dangerous complications, and more children will lose their lives before birth.
At PA Family Institute, we remain committed to exposing the truth about these dangerous drugs and advocating for policies that protect both mothers and babies. As this issue unfolds, we will continue to share the stories and data the abortion lobby works so hard to keep hidden.
Our message to the FDA is simple: women deserve better than abortion pills.



I don’t believe in abortions, unless the mother will most likely die
during childbirth. Perhaps we should allow the anti-baby pill, which I myself did not believe in. I was married and had 4 children. All were healthy and I had no complications. I have to say the Rhythm Method does not work!!
Years ago mother’s had 10 to 15 children, when the mother’s did not have part-time or fulltime jobs, however with 10 or more children the mother had enough to do raising the children and taking care of the household. Today everybody wants to travel and have the latest gadgets, so women have to work. I guess this makes the economy work. There are not enough people who would take care of somebody else’s children.
I want to add that I read a letter from a great aunt that stated that her mother died during childbirth and she heard her screaming in pain across the valley, since there was no help. She already had 10 children. I would not want to wish that on anybody. There was no mother to take care of the children so they were all split up among other families. Men have to be on board too and support their wives.
I hope you will keep your word and not release my name and e-mail address.